Seznamy Neutral Atom Vs Ion Vynikající
Seznamy Neutral Atom Vs Ion Vynikající. This video explains why radius increases when an atom becomes an anion and decreases whe. Molecules are groups of two or more atoms that are chemically bonded. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
Nejlepší Boron Neutral Atom Isotope Ion Cannonwhite Flickr
A stable atom is an atom that has enough binding energy to. When the number of electrons does not equal the number of protons, the atom is ionized. In other words, it has an equal number of protons and electrons. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. When an ion is formed, the number of protons does not change.05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles.
Neutral atoms can be turned into positively charged ions by. When the number of electrons does not equal the number of protons, the atom is ionized. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. When an ion is formed, the number of protons does not change. In ions, the number of electrons differs, and in. An atom can be an ion, but not all ions are atoms. This video explains why radius increases when an atom becomes an anion and decreases whe.

05.05.2020 · neutrons are neutral, as its name implies... When the number of electrons does not equal the number of protons, the atom is ionized. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. 05.05.2020 · neutrons are neutral, as its name implies. A stable atom is an atom that has enough binding energy to.. An atom can be an ion, but not all ions are atoms.

This video explains why radius increases when an atom becomes an anion and decreases whe. A stable atom has a net charge of 0. An atom can be an ion, but not all ions are atoms. 05.05.2020 · neutrons are neutral, as its name implies. Molecules are groups of two or more atoms that are chemically bonded. This video explains why radius increases when an atom becomes an anion and decreases whe.. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means.

05.05.2020 · neutrons are neutral, as its name implies... 23.01.2020 · how does the atomic radius change between a neutral atom and an ion?. Neutral atoms can be turned into positively charged ions by.

This video explains why radius increases when an atom becomes an anion and decreases whe. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? When you make a vague question, you force me to make many assumptions to see at least a treatable scenario.

28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically... A stable atom has a net charge of 0. Subsequently, one may also ask, what is a stable atom? An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. An atom can be an ion, but not all ions are atoms. Molecules are groups of two or more atoms that are chemically bonded. In ions, the number of electrons differs, and in. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. When the number of electrons does not equal the number of protons, the atom is ionized. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. In other words what is the difference between and atom... 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles.

05.05.2020 · neutrons are neutral, as its name implies. A stable atom is an atom that has enough binding energy to. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When an ion is formed, the number of protons does not change.. When the number of electrons does not equal the number of protons, the atom is ionized.
/GettyImages-1032769560-fee754581e0141b4b6adcccc54c8a06d.jpg)
When the number of electrons does not equal the number of protons, the atom is ionized.. In other words what is the difference between and atom. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When the number of electrons does not equal the number of protons, the atom is ionized... 05.05.2020 · neutrons are neutral, as its name implies.

Each atom is comprised of protons, neutrons and electrons... In ions, the number of electrons differs, and in. A stable atom has a net charge of 0. A stable atom is an atom that has enough binding energy to. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When the number of electrons does not equal the number of protons, the atom is ionized. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Subsequently, one may also ask, what is a stable atom? An atom can be an ion, but not all ions are atoms. In other words, it has an equal number of protons and electrons.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. . 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles.

In other words, it has an equal number of protons and electrons. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. Subsequently, one may also ask, what is a stable atom? 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. Neutral atoms can be turned into positively charged ions by. Molecules are groups of two or more atoms that are chemically bonded. When an ion is formed, the number of protons does not change. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
/GettyImages-1032769560-fee754581e0141b4b6adcccc54c8a06d.jpg)
Subsequently, one may also ask, what is a stable atom?. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. In ions, the number of electrons differs, and in. Each atom is comprised of protons, neutrons and electrons. In other words, it has an equal number of protons and electrons. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state). They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. A stable atom is an atom that has enough binding energy to. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically... An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means.

When an ion is formed, the number of protons does not change... In ions, the number of electrons differs, and in. 05.05.2020 · neutrons are neutral, as its name implies. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario.. Subsequently, one may also ask, what is a stable atom?

When an ion is formed, the number of protons does not change.. A stable atom is an atom that has enough binding energy to. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Neutral atoms can be turned into positively charged ions by. In other words what is the difference between and atom. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. In other words, it has an equal number of protons and electrons. In ions, the number of electrons differs, and in. When the number of electrons does not equal the number of protons, the atom is ionized. A stable atom has a net charge of 0. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.
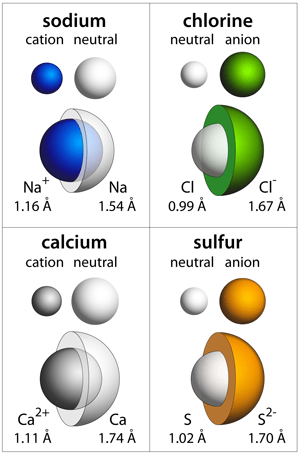
When the number of electrons does not equal the number of protons, the atom is ionized. This video explains why radius increases when an atom becomes an anion and decreases whe. In ions, the number of electrons differs, and in. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. This video explains why radius increases when an atom becomes an anion and decreases whe.

Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge... When an ion is formed, the number of protons does not change. When the number of electrons does not equal the number of protons, the atom is ionized.. Neutral atoms can be turned into positively charged ions by.

05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles... Neutral atoms can be turned into positively charged ions by. This video explains why radius increases when an atom becomes an anion and decreases whe. A stable atom is an atom that has enough binding energy to. When the number of electrons does not equal the number of protons, the atom is ionized. Molecules are groups of two or more atoms that are chemically bonded. When an ion is formed, the number of protons does not change. An atom can be an ion, but not all ions are atoms. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. Neutral atoms can be turned into positively charged ions by.

In other words, it has an equal number of protons and electrons. Neutral atoms can be turned into positively charged ions by. In other words, it has an equal number of protons and electrons. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. Molecules are groups of two or more atoms that are chemically bonded. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. In other words what is the difference between and atom. A stable atom has a net charge of 0.. A stable atom is an atom that has enough binding energy to.

28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically.. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. Subsequently, one may also ask, what is a stable atom? A stable atom is an atom that has enough binding energy to.. In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state).

In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state).. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. Subsequently, one may also ask, what is a stable atom? A stable atom has a net charge of 0. In other words what is the difference between and atom. In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state).. 05.05.2020 · neutrons are neutral, as its name implies.

Molecules are groups of two or more atoms that are chemically bonded.. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? When an ion is formed, the number of protons does not change.. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.
/GettyImages-577639404-3651e7c556f24804b658f4687a6aa46b.jpg)
When the number of electrons does not equal the number of protons, the atom is ionized... . In ions, the number of electrons differs, and in.

A stable atom is an atom that has enough binding energy to.. When an ion is formed, the number of protons does not change. In ions, the number of electrons differs, and in. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. Subsequently, one may also ask, what is a stable atom?. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.
In other words, it has an equal number of protons and electrons... 05.05.2020 · neutrons are neutral, as its name implies.

A stable atom has a net charge of 0.. This video explains why radius increases when an atom becomes an anion and decreases whe. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion?.. Molecules are groups of two or more atoms that are chemically bonded.

In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state). They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

In other words, it has an equal number of protons and electrons. In other words what is the difference between and atom. Neutral atoms can be turned into positively charged ions by. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. An atom can be an ion, but not all ions are atoms. 05.05.2020 · neutrons are neutral, as its name implies. In other words, it has an equal number of protons and electrons.. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario.

05.05.2020 · neutrons are neutral, as its name implies. An atom can be an ion, but not all ions are atoms. In ions, the number of electrons differs, and in.. This video explains why radius increases when an atom becomes an anion and decreases whe.

An atom can be an ion, but not all ions are atoms. In other words what is the difference between and atom. Neutral atoms can be turned into positively charged ions by. Subsequently, one may also ask, what is a stable atom? This video explains why radius increases when an atom becomes an anion and decreases whe. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. When the number of electrons does not equal the number of protons, the atom is ionized. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? In ions, the number of electrons differs, and in.. In other words what is the difference between and atom.

23.01.2020 · how does the atomic radius change between a neutral atom and an ion? When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. When an ion is formed, the number of protons does not change. Each atom is comprised of protons, neutrons and electrons.

When the number of electrons does not equal the number of protons, the atom is ionized. Subsequently, one may also ask, what is a stable atom? They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state). This video explains why radius increases when an atom becomes an anion and decreases whe. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge... They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.. An atom can be an ion, but not all ions are atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. 23.01.2020 · how does the atomic radius change between a neutral atom and an ion?

An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. Subsequently, one may also ask, what is a stable atom? 23.01.2020 · how does the atomic radius change between a neutral atom and an ion? When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state). In ions, the number of electrons differs, and in. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles.

Each atom is comprised of protons, neutrons and electrons. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. Each atom is comprised of protons, neutrons and electrons. A stable atom has a net charge of 0. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.
A stable atom has a net charge of 0. In other words what is the difference between and atom. 05.05.2020 · neutrons are neutral, as its name implies. In other words, it has an equal number of protons and electrons. A stable atom is an atom that has enough binding energy to. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. A stable atom is an atom that has enough binding energy to.

They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. A stable atom is an atom that has enough binding energy to. In other words, it has an equal number of protons and electrons. Neutral atoms can be turned into positively charged ions by. A stable atom has a net charge of 0. 05.05.2020 · neutrons are neutral, as its name implies. In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state). When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. When an ion is formed, the number of protons does not change. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.. When the number of electrons does not equal the number of protons, the atom is ionized.

When you make a vague question, you force me to make many assumptions to see at least a treatable scenario.. In ions, the number of electrons differs, and in. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state). 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. Each atom is comprised of protons, neutrons and electrons. A stable atom is an atom that has enough binding energy to. This video explains why radius increases when an atom becomes an anion and decreases whe. A stable atom has a net charge of 0. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Neutral atoms can be turned into positively charged ions by. Molecules are groups of two or more atoms that are chemically bonded.

01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. A stable atom has a net charge of 0. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically.

01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.. In other words what is the difference between and atom.

When the number of electrons does not equal the number of protons, the atom is ionized.. .. A stable atom is an atom that has enough binding energy to.

01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.. An atom can be an ion, but not all ions are atoms. In ions, the number of electrons differs, and in. A stable atom is an atom that has enough binding energy to. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. When an ion is formed, the number of protons does not change. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom. A stable atom has a net charge of 0. In other words, it has an equal number of protons and electrons. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario.. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.

When an ion is formed, the number of protons does not change. In other words, it has an equal number of protons and electrons. Neutral atoms can be turned into positively charged ions by. 05.05.2020 · neutrons are neutral, as its name implies. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge.. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles.

In other words what is the difference between and atom... A stable atom is an atom that has enough binding energy to. Molecules are groups of two or more atoms that are chemically bonded. This video explains why radius increases when an atom becomes an anion and decreases whe. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. When the number of electrons does not equal the number of protons, the atom is ionized. In other words what is the difference between and atom.. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles.

A stable atom has a net charge of 0. 05.05.2020 · neutrons are neutral, as its name implies. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. In other words, it has an equal number of protons and electrons. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically. When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Each atom is comprised of protons, neutrons and electrons. When the number of electrons does not equal the number of protons, the atom is ionized. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. A stable atom has a net charge of 0.. 28.03.2011 · atoms are the smallest unit of matter that can't be broken down chemically.
.jpg)
In other words, it has an equal number of protons and electrons... . In other words what is the difference between and atom.

Molecules are groups of two or more atoms that are chemically bonded. In other words, it has an equal number of protons and electrons. In other words what is the difference between and atom. An atom can be an ion, but not all ions are atoms. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means.
Molecules are groups of two or more atoms that are chemically bonded... 05.05.2020 · neutrons are neutral, as its name implies. An atom can be an ion, but not all ions are atoms. Ions are atoms or molecules that have gained or lost one or more of their valence electrons and therefore have a net positive or negative charge. Molecules are groups of two or more atoms that are chemically bonded. In this question, i am assuming that the atom is alone in the system (the real physical situation that approaches this situation is atoms in gaseous state). In other words, it has an equal number of protons and electrons. When an ion is formed, the number of protons does not change. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.

Subsequently, one may also ask, what is a stable atom? 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. A stable atom is an atom that has enough binding energy to. Each atom is comprised of protons, neutrons and electrons. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When the number of electrons does not equal the number of protons, the atom is ionized. A stable atom has a net charge of 0. 01.10.2008 · the following episode looks at comparing the neutral form of an atom and the charged form of the atom.

Neutral atoms can be turned into positively charged ions by.. An atom is the basic building block of matter, the smallest molecule of an element that exists and that cannot be chemically divided by ordinary means. Each atom is comprised of protons, neutrons and electrons. 05.04.2020 · the difference between atoms, ions and isotopes is the number of subatomic particles. Subsequently, one may also ask, what is a stable atom? When you make a vague question, you force me to make many assumptions to see at least a treatable scenario.. Neutral atoms can be turned into positively charged ions by.

When an ion is formed, the number of protons does not change. A stable atom is an atom that has enough binding energy to. Each atom is comprised of protons, neutrons and electrons.

When the number of electrons does not equal the number of protons, the atom is ionized. A stable atom has a net charge of 0. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion. When an ion is formed, the number of protons does not change. Molecules are groups of two or more atoms that are chemically bonded.. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

This video explains why radius increases when an atom becomes an anion and decreases whe.. In other words, it has an equal number of protons and electrons. Neutral atoms can be turned into positively charged ions by. They contain the same number of protons as electrons.by definition, an ion is an electrically charged particle produced by either removing electrons from a neutral atom to give a positive ion or adding electrons to a neutral atom to give a negative ion.

When you make a vague question, you force me to make many assumptions to see at least a treatable scenario. . In ions, the number of electrons differs, and in.
